Do You Have to Know Cranial Nerves for Ati Teas
May 17, 2020
Getting set up to take the ATI TEAS? We've got the science section covered in this mail service. Read top tips and key facts you need to know.
No affair what phase of studying you're in or how many times you've attempted the exam, y'all should read these 20 ATI TEAS Science section review tips. We've gathered key information as to what topics are on the scientific discipline section. Be sure you sympathize these topics earlier taking the official exam. It's easy to tell yourself that yous understand something complex, but are you really sure? A neat way to exam yourself is to explain the concept to a friend or family member. If yous can teach them about the topic, you know you've fully grasped the concept.
Take note of these 20 items and make certain you lot've fully mastered them before yous sit for the official ATI TEAS test. Proceed reading to learn how to pass ATI TEAS Science section with ease.
i. Scientific Method / Scientific Reasoning
Memorize the scientific method steps (in order) and know specific examples of each pace.
- Problem identification
- Question asking
- Hypothesis development
- Data drove and experimentation
- Analysis
- Conclusion
2. Concrete States of Affair
Empathize states of matter (solid, liquid, gas). Know how a change in state may change the pressure or volume of the affair.
iii. Chemical Properties of Water
Learn what temperatures water freezes at and boils at.
four. Kinetic and Potential Energy
Understand what each means and make certain you can recognize an case of each.
The corporeality of energy associated with an object'due south motion may exist quantified through a calculation of its kinetic energy (KE), or energy of move. Any increase in an object'south velocity (in units of meters per second in the metric system) will result in a dramatic increase in the object's KE. Specifically, any doubling of the velocity volition cause the KE to increase by a factor of four times.
The corporeality of stored energy in an object may exist quantified through a calculation of its potential energy (PE), or stored free energy. Energy may be stored in several means, every bit in a common bombardment cell or the gasoline in a fuel tank. The Globe's gravity may also store free energy when an object is held at a certain height. Specifically, any doubling of the meridian volition too double the PE.
KE and PE have a unique connection. PE tin be used to produce an object's motion, which is KE. Both forms of energy have a dynamic interplay through the conservation of energy. Conservation of energy occurs when a full constant of energy is maintained by the conversion of energy between kinetic and potential. If a system is considered airtight and isolated (no mass or energy is inbound or leaving), then free energy may only exist converted from i form of energy to another. Generally, the energy of movement (KE) may exist increased, merely only at the expense of converting stored energy (PE). The reverse conversion may too occur (kinetic to potential), but the total energy for the arrangement must remain stock-still. In short, the Law of Conservation of Free energy says that energy is non lost but rather transferred back and along between KE and PE. Given a fixed corporeality of total energy in a system, an increment in KE will event in a subtract in PE (and vice versa), but the total amount of free energy will remain the same.
5. Chloroplasts
Plants comprise chloroplasts, which are organelles that contain chlorophyll. Chlorophyll allows the capture of sunlight to exist used for the production of glucose during photosynthesis. Chloroplasts have many structural similarities to mitochondria, but found cells need both mitochondria and chloroplasts.
six. Jail cell Walls
Know the anatomy of both plant and animal cell walls.
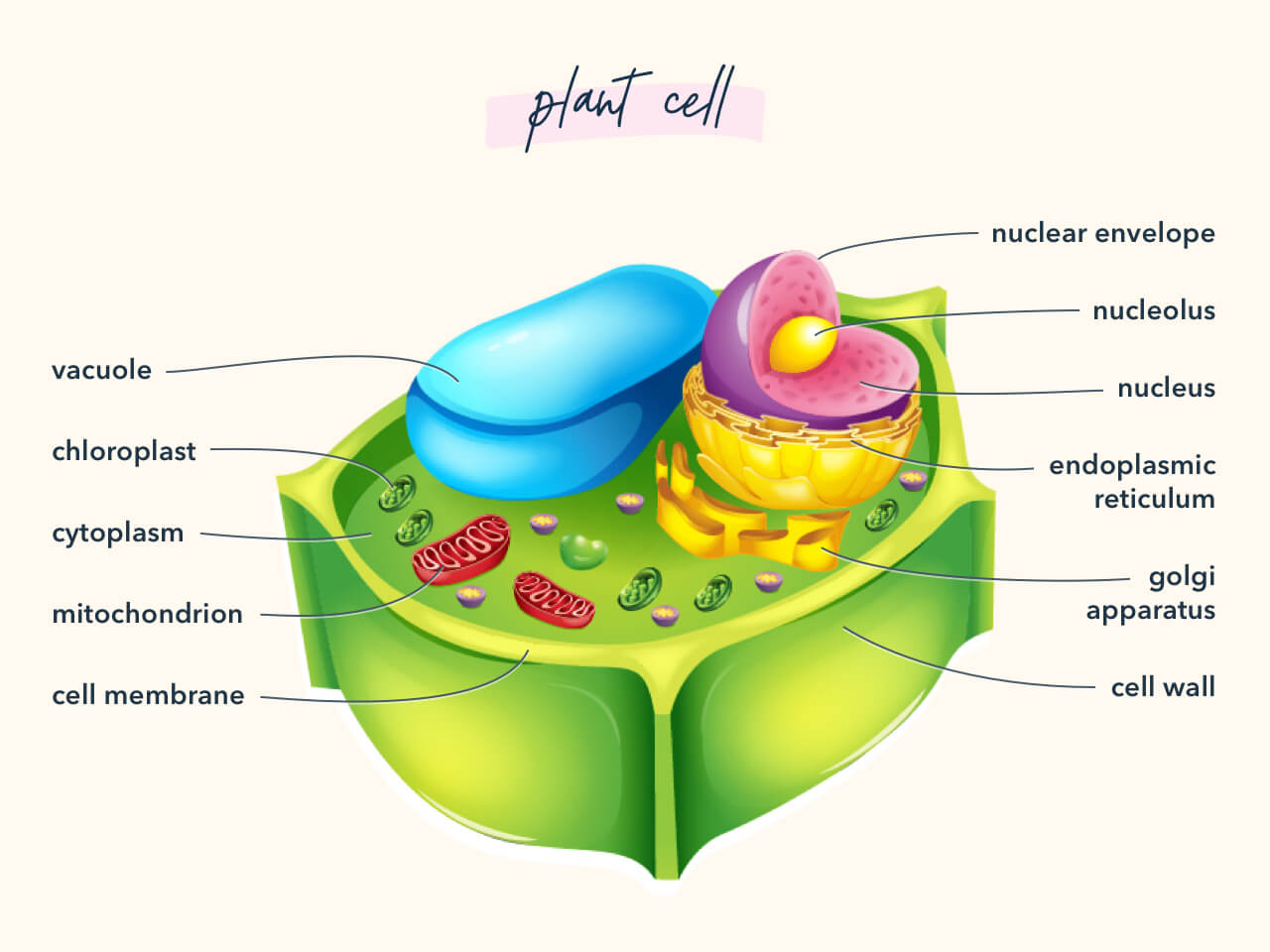
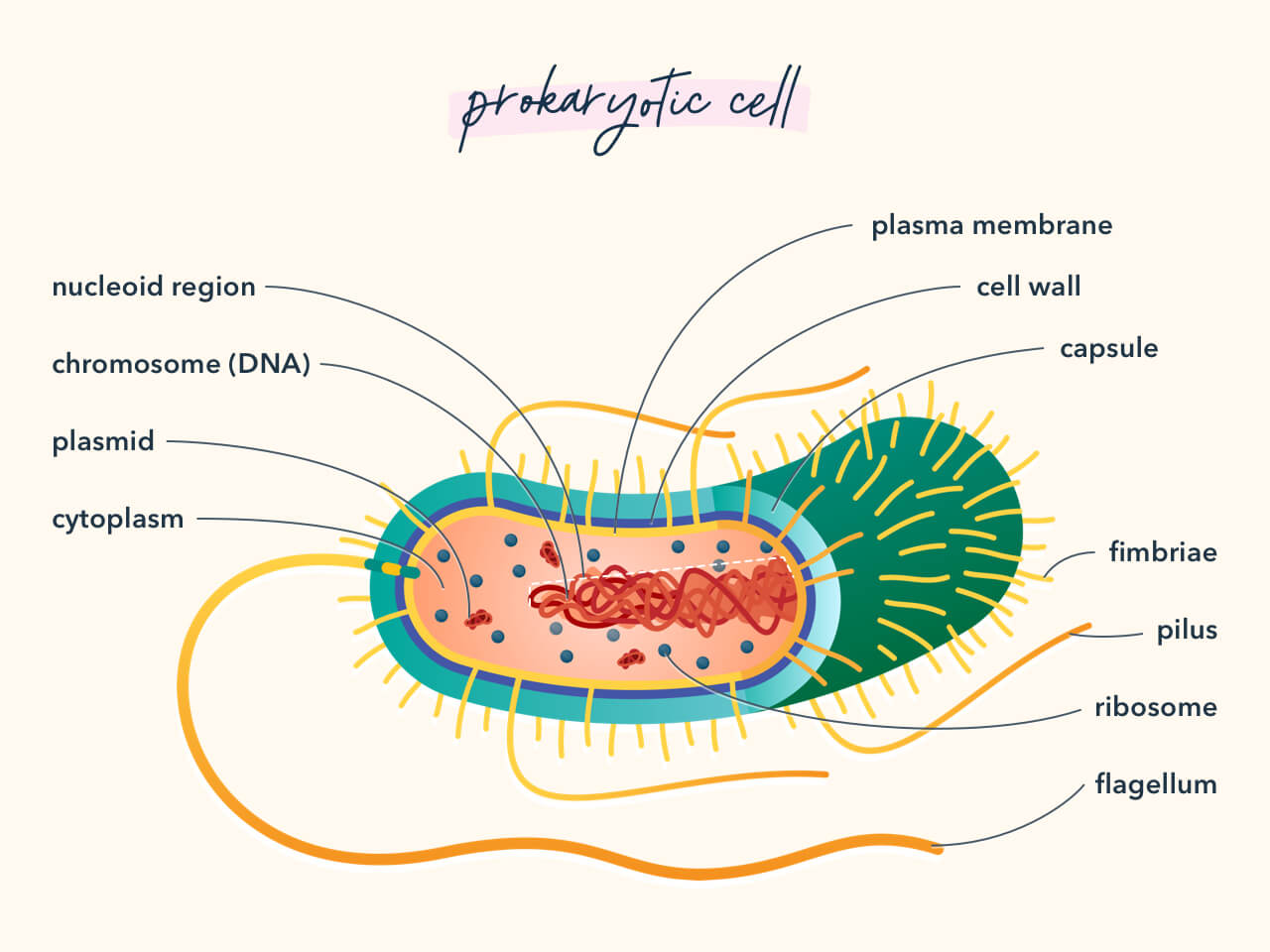
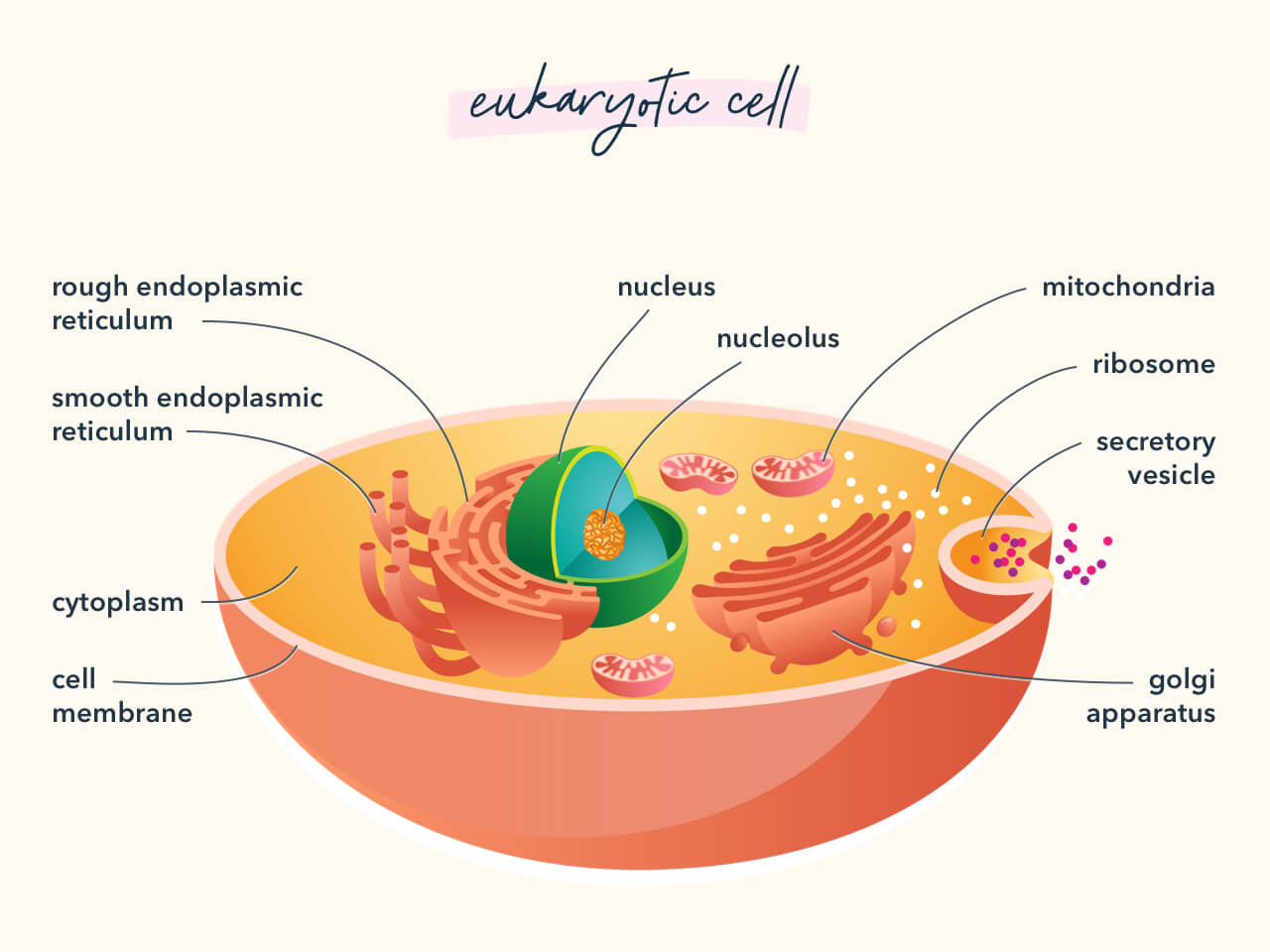
7. Mutation vs. Adaption
Mutations are changes in Deoxyribonucleic acid sequences (e.m., species variations). Because organisms have environmental pressures such as food, water, space, predation, etc., if a mutation/variation causes a positive result that makes the organism easier to withstand environmental pressures, it is said to be an adaptation.
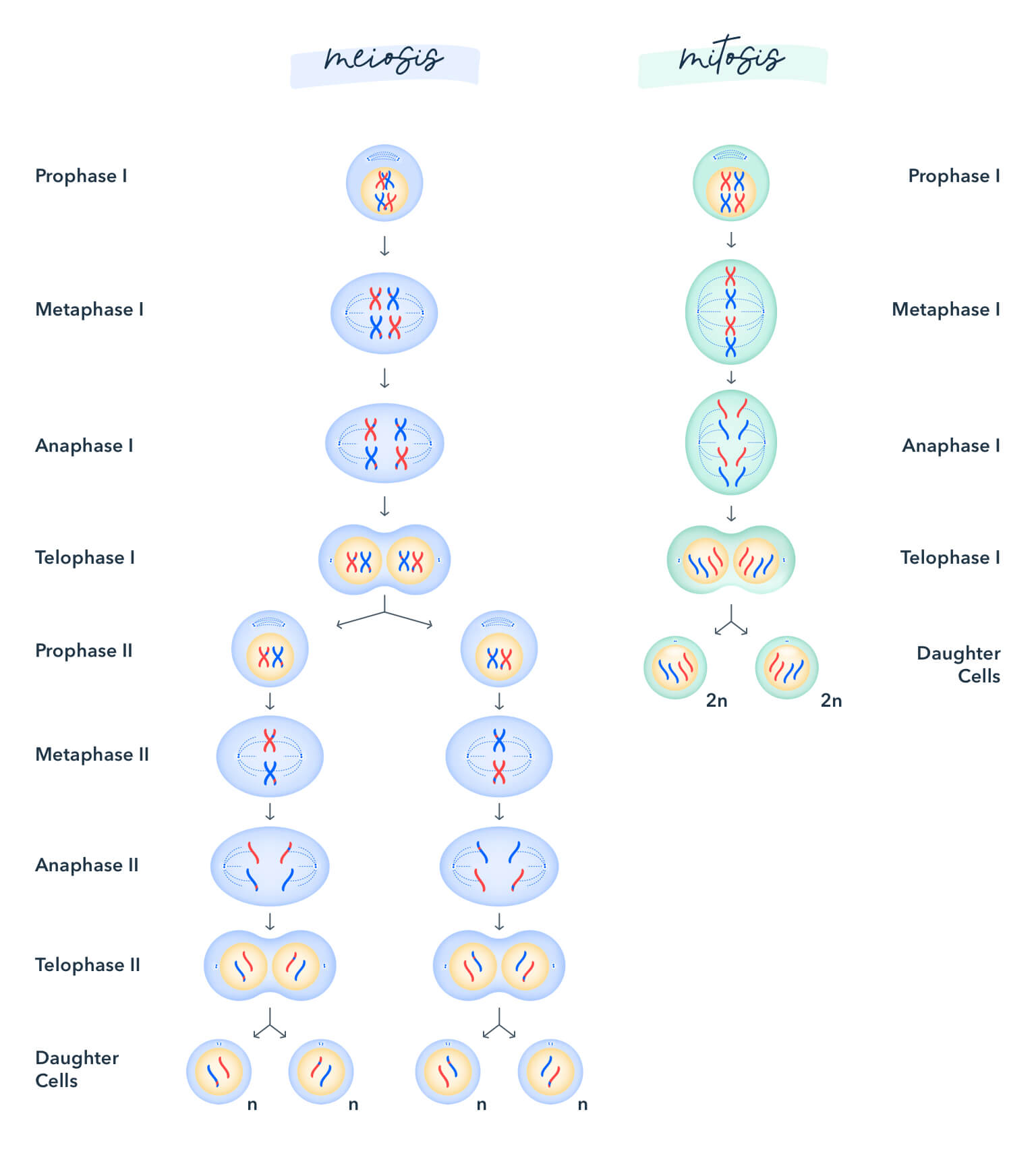
eight. Mitosis and Meiosis
Understand all phases of each and what is happening.
Mitosis: Chromosomes in a cell nucleus are separated into two identical sets of chromosomes, each in its own nucleus.
Meiosis: A specialized type of cell segmentation which reduces the chromosome number past half. This procedure occurs in all sexually reproducing eukaryotes (both single-celled and multicellular) including animals, plants, and fungi.
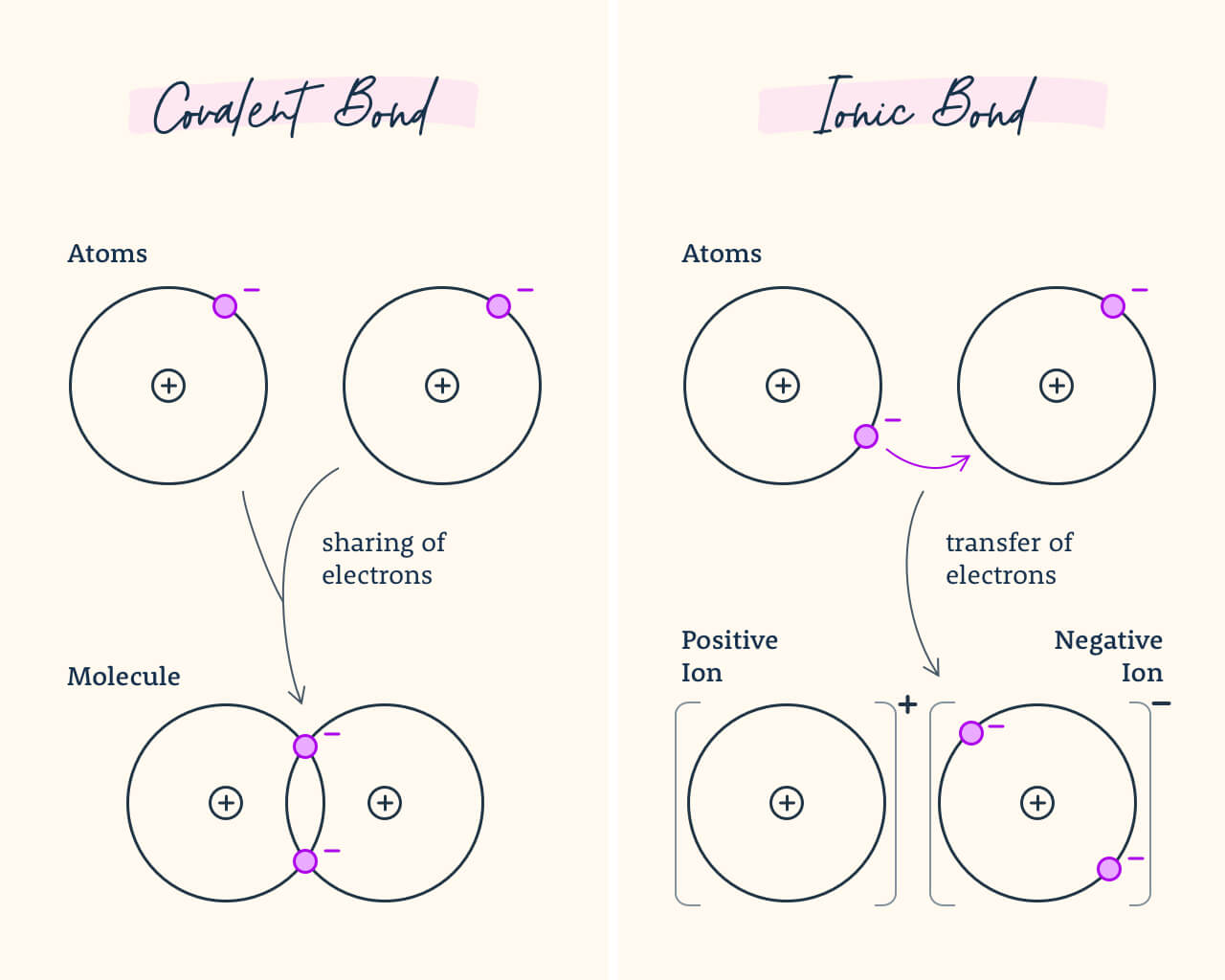
9. Bonds
An ionic bond is a chemic bail in which i cantlet loses an electron to form a positive ion and the other atom gains an electron to form a negative ion. A covalent bail is a chemical bond that involves sharing a pair of electrons between atoms in a molecule.
ten. Mendel's Laws / Punnet Squares
Understand Punnett Squares and ensure that you can set one up properly. Know the difference between heterozygous, homozygous, recessive, and dominant and how they each fit into the equation.
eleven. Phenotype and Genotype
Phenotypes are the physical expressions of genetic traits. In plants, phenotypes are things like seed shape and color. In humans, phenotypes are characteristics like brownish pilus and green eyes.
A genotype is an organism's underlying genetic makeup or lawmaking. Information technology is a blueprint for building and maintaining all structures within the cells of the body, from small proteins to metabolic activities. The Dna within genes codes for proteins that determine hereditary traits that will exist passed on between generations. Interactions betwixt the genotype and the environment affect the phenotype of the organism.
Organisms can have different genotypes, but the same phenotype (meaning organisms expect the same).
12. Nucleic Acids, Dna, and RNA
The job of nucleic acids is to store and transmit hereditary information. A nucleic acid is a chain of nucleotides that consists of a pentose, a phosphate group, and a nitrogenous base. A pentose is a type of carbohydrate and a phosphate group is a molecule in the backbone of Dna and RNA that links adjoining bases together. A nitrogenous base of operations is a molecule found in Dna and RNA that encodes the genetic information in cells. There are five types of nitrogenous bases: adenine, cytosine, guanine, thymine, and uracil. Adenine, cytosine, and guanine are found in both Dna and RNA, while thymine is unique to Deoxyribonucleic acid and uracil to RNA.
DNA is most often seen in a structure known equally the double helix. This circuitous is able to form because weak bonds are able to form between the hydrogen atoms and oxygen or nitrogen atoms betwixt bases in the complementary strands of DNA. This kind of weak bond is called a hydrogen bond because one partner in the bond is always a hydrogen atom. In DNA, adenine (A) always pairs with thymine (T), and guanine (Chiliad) e'er pairs with cytosine (C).
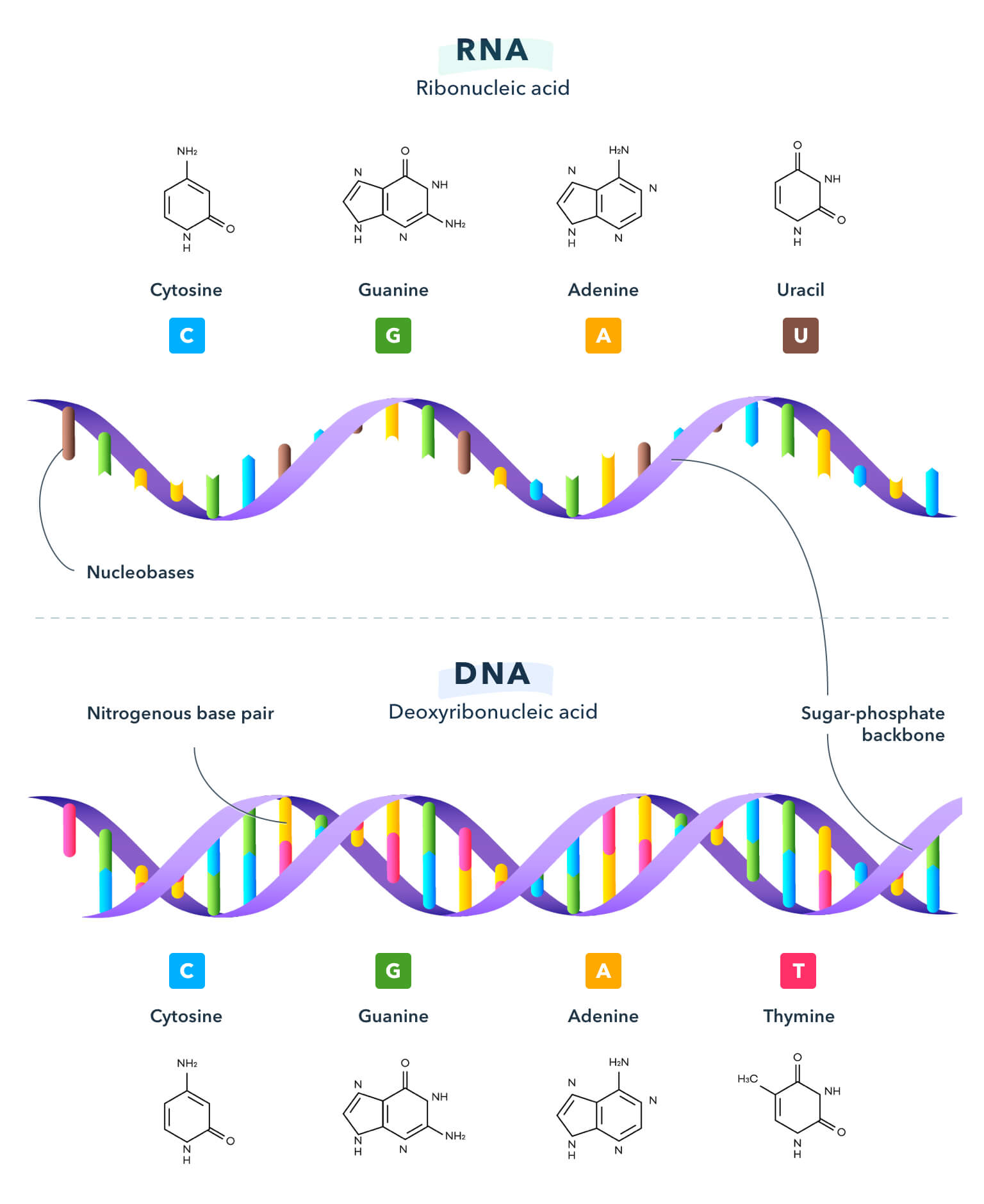
Deoxyribonucleic acid is the genetic blueprint of the prison cell and RNA can be thought of equally the messenger within the cell. The message stored in the bases of DNA must be transferred to the ribosomes to make proteins. Cells copy the instructions in the DNA into RNA (transcription) and send the messenger RNA to the ribosomes. So, proteins are made by ribosomes from the data and sent out to the entire jail cell. This process of protein production from messenger RNA is called translation. Dna, RNA, and ribosomes work hand in hand to produce the proteins necessary for life in cells.
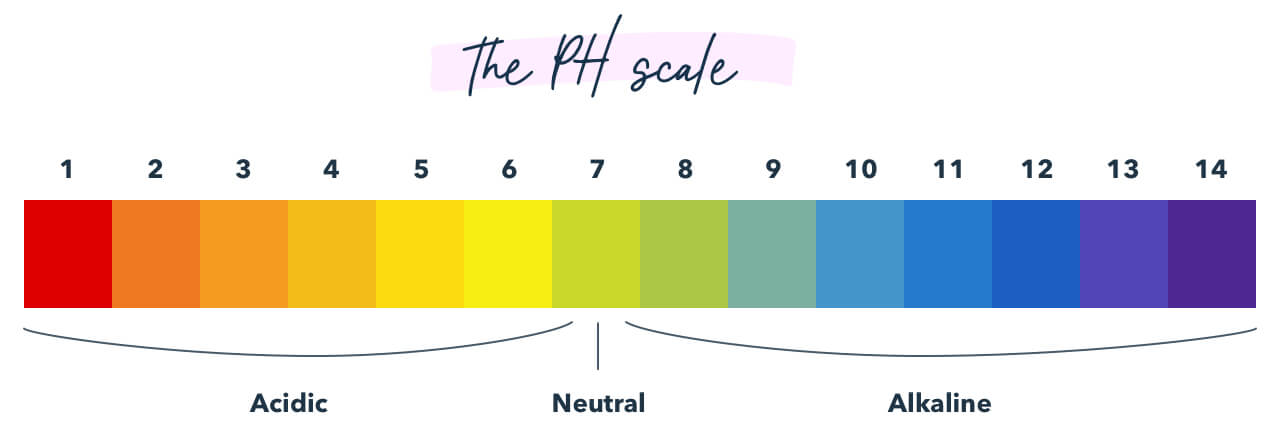
13. pH Residue
Know the pH calibration and empathize what may happen if something is added to information technology. (Example: What may raise or lower the pH of blood?)
14. Periodic Table
Know everything about the periodic table!
- Atomic number of an element
- Diminutive mass of an chemical element
- How many protons, electrons, and neutrons are in a given element
- What the rows hateful
- What the columns hateful
- Which elements are more likely to have ionic/covalent bonds
- Electron configuration
15. Balancing a Chemical Equation
You absolutely MUST know how to balance a basic chemic equation.
Video by Khan Academy
16. Cell Differentiation
Cell differentiation is how generic embryonic cells become specialized cells. This occurs through a procedure called cistron expression. Gene expression is the specific combination of genes that are turned on or off (expressed or repressed), and this is what dictates how a cell functions.
- The endoderm creates the tum, colon, liver, pancreas, urinary float, lining of the urethra, epithelial parts of trachea, lungs, throat, thyroid, parathyroid, the intestines.
- The Mesoderm forms the skeletal muscle, the skeleton, the dermis of the skin, connective tissue, the urogenital organization, the heart, claret (lymph cells), and the spleen.
- The Ectoderm forms the key nervous system, the lens of the eye, cranial and sensory, the ganglia and fretfulness, pigment cells, caput connective tissues, the epidermis, hair, and mammary glands.
17. Chromosomes, Genes, and Alleles
Know what they are, how they chronicle to each other, and how they affect organisms.
18. Biological Classification Organisation
Know the eight parts of the biological classification system. Domain, Kingdom, Phylum, Class, Society, Family, Genus, Species. You can recollect this with a clever mnemonic: 'Dumb Kids Playing Chess On Freeway Get Smushed'
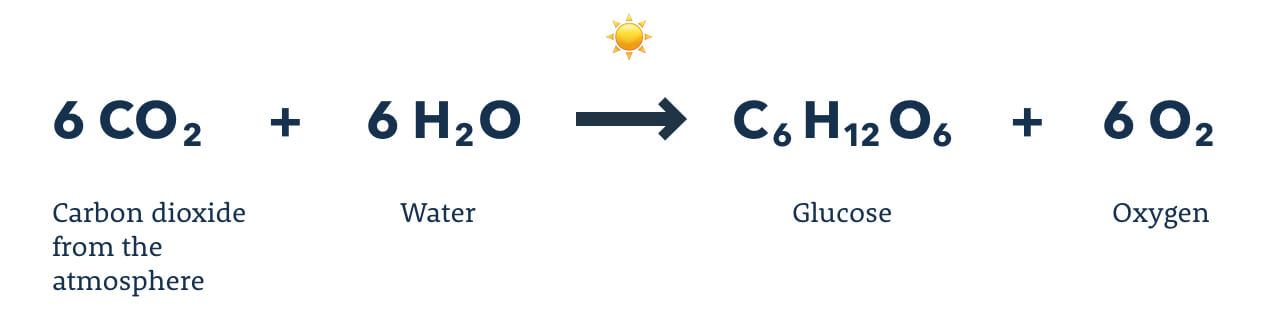
xix. Photosynthesis
Photosynthesis is the procedure carried out past green plants, greenish algae, and certain bacteria, in which the energy from sunlight is trapped by the green pigment chlorophyll and used for the synthesis of glucose. These organisms are able to acquit out photosynthesis due to the specialized organelle called a chloroplast. In the chloroplast, carbon dioxide, water, and energy from the lord's day are used to produce adenosine triphosphate (ATP), which is a cellular fuel that provides the energy to produce glucose from carbon dioxide and h2o, liberating oxygen in the process. An organism that is able to produce its ain nutrient is termed an autotroph, and nigh autotrophs use photosynthesis to live. In contrast, heterotrophs are organisms that cannot produce their own food.
xx. Anatomy and Physiology
Because anatomy and physiology is such a broad subject area, we advise a diversity of topics. Check out Pocket Prep's Nursing School app for specific A&P help.
- Anatomy of the centre valves and the path of claret through the heart
- Anatomy of the lungs and where oxygen exchange occurs
- Sections of the brain and the responsibleness of each
- Types of tissue, where they are located, and what each type does
- Digestive arrangement
- Functions of the liver, pancreas, and spleen (what system or systems each vest to)
- Lymph organization
- Nervous system
- Kidney structure and function
- Anatomical directions every bit they utilise to specific examples
- Functions of the thyroid and parathyroid individually and together
- Immune organisation
Some of the above data is from the ATI Testing TEAS Written report Guide.
gonzaleznotneinme.blogspot.com
Source: https://www.pocketprep.com/posts/how-to-pass-the-ati-teas-science-section/
0 Response to "Do You Have to Know Cranial Nerves for Ati Teas"
Post a Comment